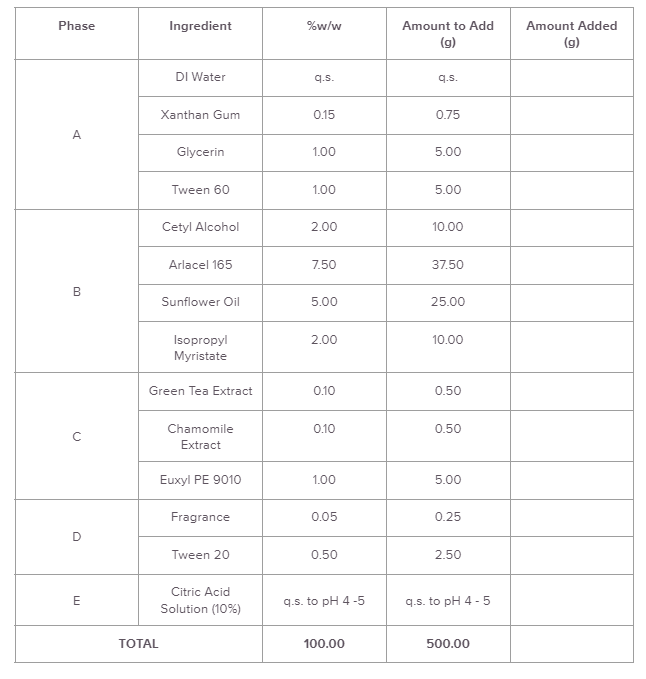
We measure everything in formulation using w/w% (weight-per-weight). This is why you need a scale to accurately measure your ingredients.
A formula is written like this:
Process:
Add DI Water to main vessel.
Disperse Xanthan Gum in Glycerin. Disperse mixture in water.
Add Tween 60.
Heat Phase A to 70 - 75 °C.
Heat Phase B to 70 - 75 °C.
Add Phase B to Phase A with homogenization.
Cool batch to <35 °C.
Add Phase C.
Premix Phase D. Add to batch.
q.s. pH to 4 - 5 as necessary.
Let’s break down what each column means and how to read the processing instructions.
Phase
You can’t put everything in a beaker, mix it, and achieve your end formulation. Ingredients are grouped into different “phases” based on the following factors:
Solubility (e.g., water-soluble ingredients are mixed first with water, oil-soluble ingredients are mixed first with oil, etc.)
Processing temperature (e.g., you don’t want to add temperature-sensitive ingredients at a hot temperature, you may have to melt some waxes, etc.)
Mixing factors (e.g., it’s better to disperse xanthan gum in a glycol before adding it to water so that it doesn’t clump in the water)
As you become more familiar with different formula types, you’ll have an easier time understanding why certain ingredients are grouped together in processing instructions.
Ingredient
This is the column where you list out each ingredient by trade name or INCI or both.
% w/w
% w/w (read as “percent weight-per-weight” or “percent weight-weight”) is the metric we use to write cosmetic formulas. We do not use tablespoons, cups, etc. in the lab. When measuring ingredients weight-per-weight, you are referring to the percentage of the weight a formula contains. For example, if you are to make a 100 g batch of the formula above, you’d see that 1.00% of the formula is glycerin.
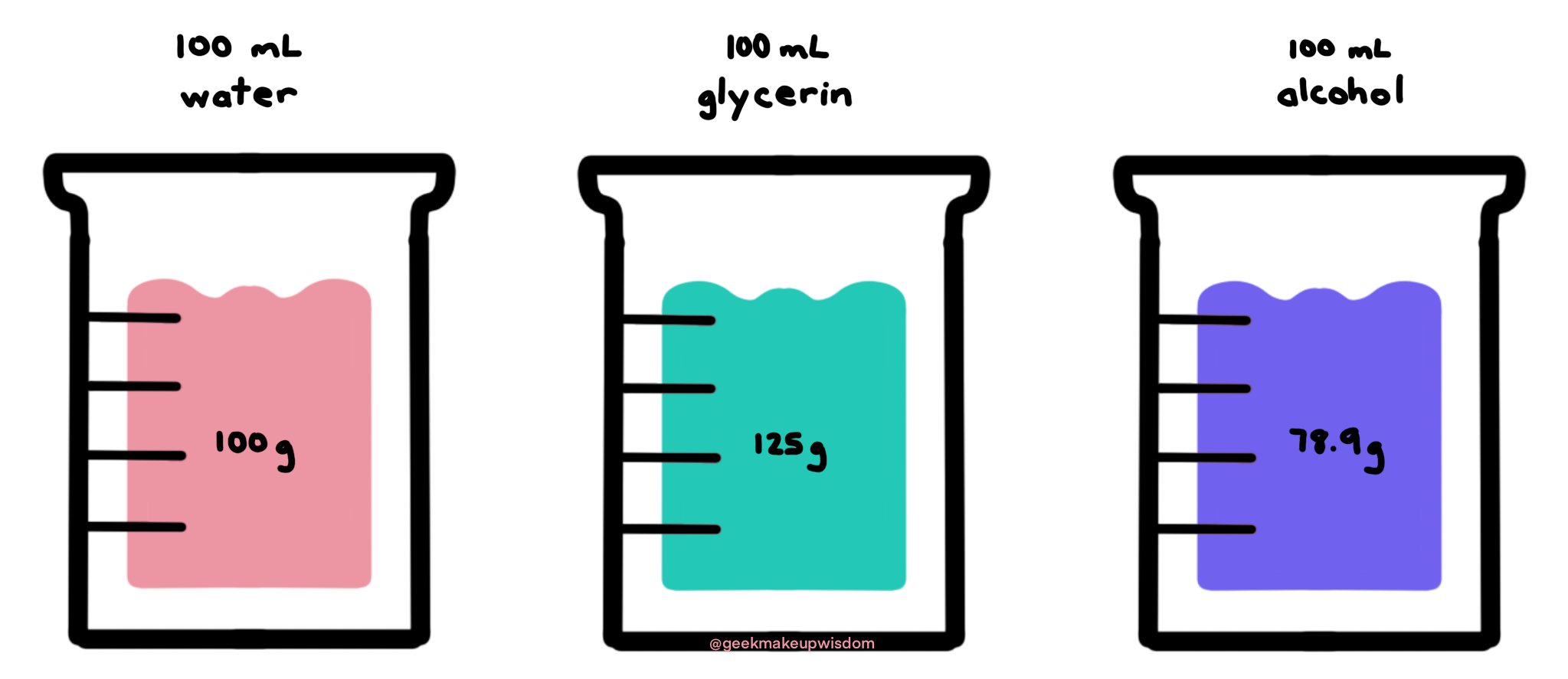
We don’t use volume because every ingredient has a different density. For example, 100 mL of water is 100 g because the density of water is 1 g/mL. 100 mL of glycerin is 125 g because the density of glycerin is 1.25 g/mL. 100 mL of dehydrated alcohol (ethanol) is 78.9 g because the density of dehydrated alcohol is 0.789 g/mL.
q.s.
q.s. stands for qauntum satis in Latin, which means the amount which is enough. When you “q.s.” an ingredient in a formula, it means you add the remaining amount of the q.s.’d ingredient in a formula. In most formulas, this will almost always be water. To calculate how much water you need to add in the example formula:
Amount to Add (g)
We use this column to determine how many grams of an ingredient we’re going to add depending on our batch size. In the example formula, we will make a 500 g batch size. If we are to add 1% of glycerin to a 500 g batch size, then we need to add 5 g of glycerin to the formula:
For the sake of another example from the formula, to calculate the amount of green tea extract to add you would say:
With this equation in mind, I’ll usually shortcut in my mind to 1% x 5 = 5 g glycerin since the zeros in the 500 g and 100% cancel each other out to 5.
Amount Added (g)
This column is how much you actually put in your formula as you’re making it. This is very important to keep track of while you’re making your batch so that if things go sideways, you can review what you did to make sure you didn’t accidentally add too little or too much of an ingredient.
It can be kind of difficult to write things and take notes as you make your formulas, but it’s important to do. I try to pre-weigh everything in beakers, weigh boats, and weigh papers as much as I can so I have less to write when I’m actually putting everything together.
If you’re new to writing formulas, I hope this was helpful for you. Leave a comment if you have any questions!