This week, I'm going to present to you some of the most common chemical structures in cosmetics. The point of this series is to prep you for my upcoming spring e-course (sign up HERE to be alerted to updates!) If you haven't taken organic chemistry yet, you may not be familiar with all of the chemical nomenclature of the different ingredients on a label. Thus, I decided to put this series of blog posts together to define some of these definitions and make understanding cosmetic chemistry a smoother transition. If you've already taken organic chemistry, you should be able to recognize most of these structures.
I have compiled a list of 35 structures that are common in cosmetics. From today until Friday, we will go over a few at a time.
Before we dive into structures, there are some vocabulary I want you to know that is necessary for understanding these blog posts:
- Substituent - a type of functional group (chemical group) that is attached to a carbon chain or cyclical carbon structure
- Functional group - a common type of chemical group, i.e. -OH is the functional group "hydroxyl"
- R - letter used to define an arbitrary substituent attached to the rest of the molecule. These "R-groups" are what define a specific molecule.
- Saturated - carbons that are singly bonding
- Unsaturated - carbons that have double or triple bonding
- Lone pair - two valence electrons on an atom that are not part of a bond
- Oxides - contain at least one oxygen (O) in its chemical formula
- Phosphorous compounds - contain a phosphorous atom (P)
- Polymer - molecule with repeated units
All righty, let's dive in! Today we will go over alkanes, alcohols, aldehydes, ethers, amides, amines, amino acids and benzene.
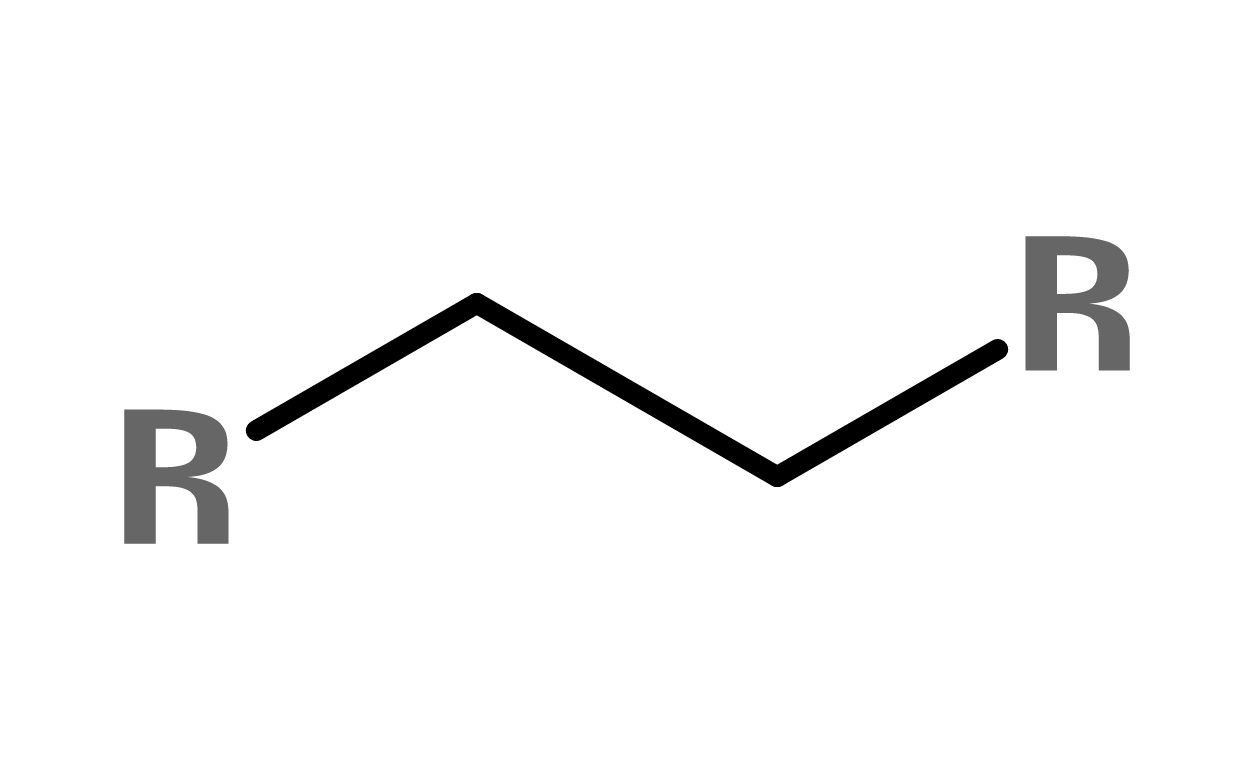
1. ALKANE (ALKYL)
A chain of C's (carbons) and H's (hydrogens), they consist of only saturated carbons. For example, isobutane is a common hydrocarbon gas used as a propellant in aerosol products.
Figure 1. General structure of an alkane
Figure 2. Isobutane
2. ALCOHOL
An alkyl chain with a hydroxyl (-OH) functional group. In cosmetics, low molecular weight alcohols are often used a solvents or astringents. Isopropyl alcohol is often used as a 70% solution for cleaning lab equipment.
Figure 3. General structure of alcohol
Figure 4. Isopropyl alcohol
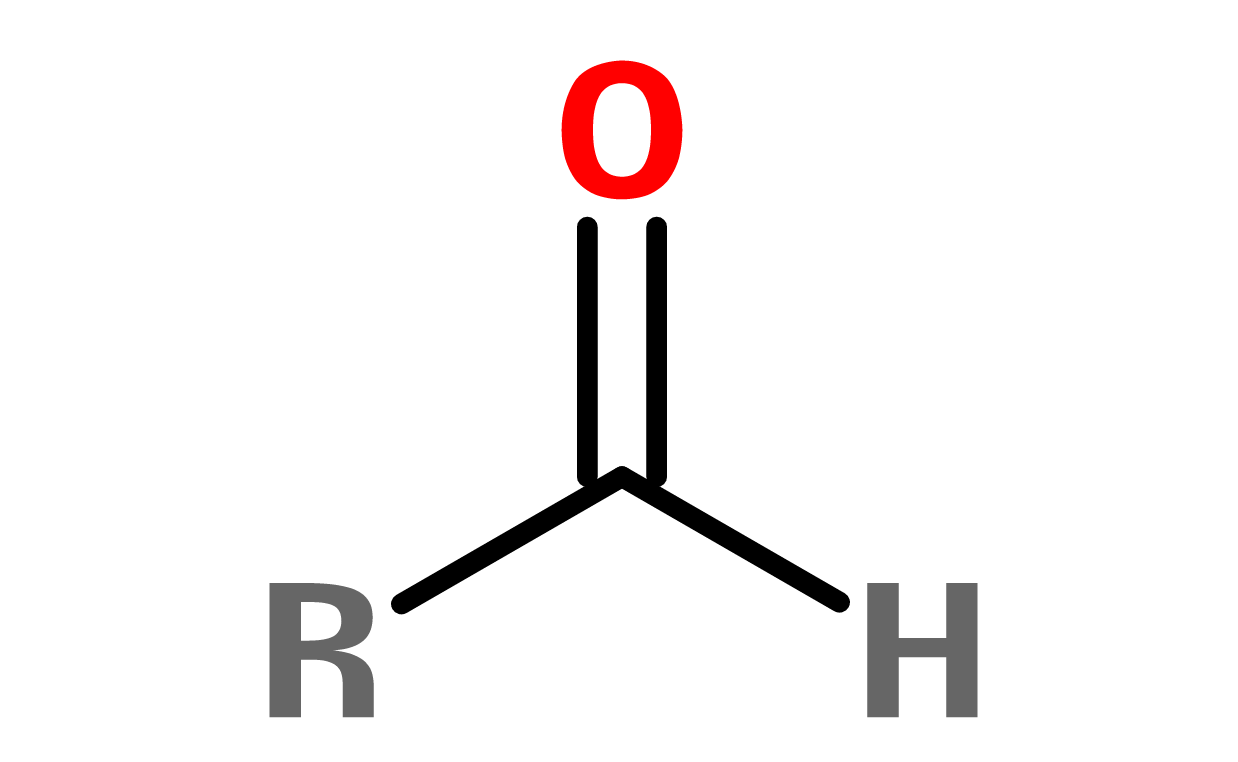
3. ALDEHYDES
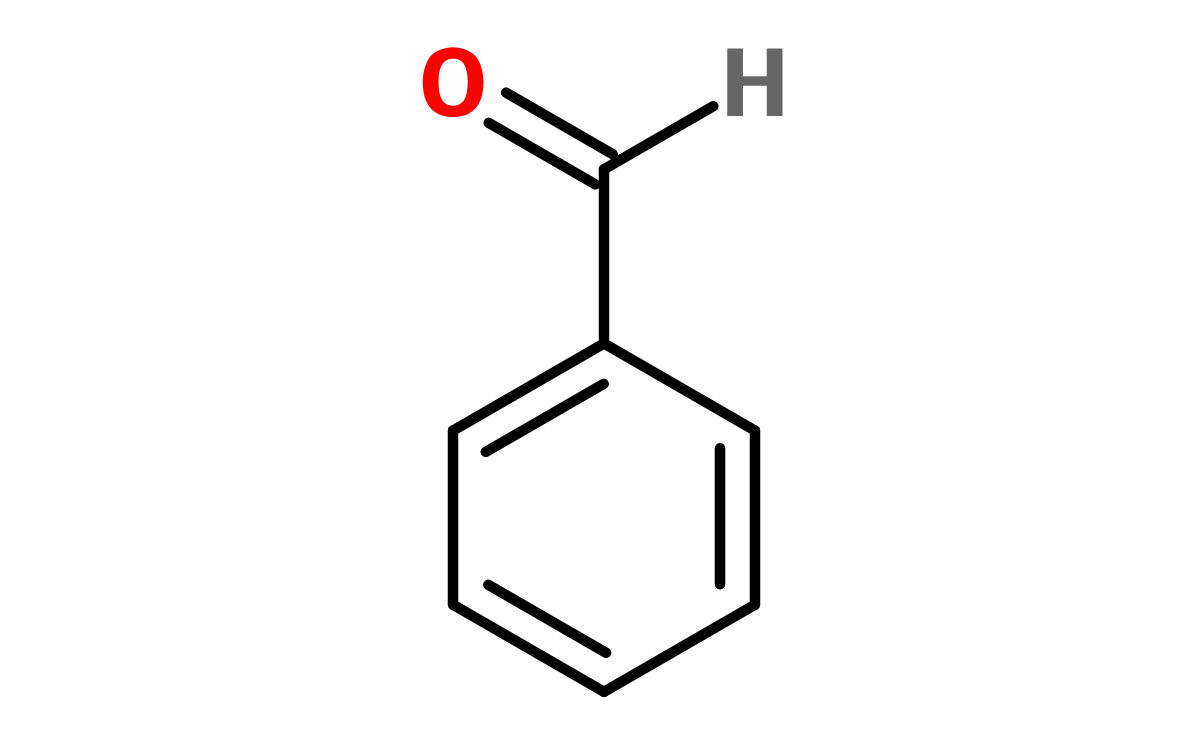
A functional group with a C double-bonded to an O (oxygen) and singly bonded to an -OH. In general, aldehydes don't have a wide range of use in cosmetics. If they are, they usually act as preservatives or gemacides. Aromatic (see benzene below) aldehydes are common structures in fragrance and flavor ingredients. Benzaldehyde is a common component of essential oils, often noted to smell like bitter almonds
Figure 5. General structure of an aldehyde
Figure 6. Benzaldehyde
4. ALKOXY GROUP (ETHER)
A molecule consisting of a C single bonded to O. Alkoxylated molecules like alkoxylated alcohol, alkoxylated amides, alkoxylated amines and alkoxylated carboxylic acids are often used as surfactants, emulsifiers, solubilizers and conditioners. In addition, alkoxylated carboxylic acids can act as emollients or suspending agents. Laureth-23 is a common example of an alkoxylated molecule. It acts as a surfactant that can be used in a variety of cleansing products
Figure 7. Alkoxy groups
Figure 8. Laureth-23
5. AMIDES
A functional group with a C double-bonded to an O and singly bonded to an -NH2. Amides are usually moisturizers, preservatives, surfactants or foam boosters in cosmetics. Cocoamide DEA is a common foaming agent in cleansing products.
Figure 9. General structure of an amide. The R-groups can be different or the same.
Figure 10. Cocoamide DEA
6. AMINES
A functional group in which an N bonds to a combination of H's and R-groups along with a lone pair in its orbital. These structures are not common in cosmetics due to their malodor. You can find species like aromatic diamines and aminophenols in hair dye, though. One of the most common amines is triethanolamine (TEA), which is a common base used to neutralize carbopols.
Figure 11. Types of amines--the R groups can be the same or different.
Figure 12. Triethanolamine (TEA)
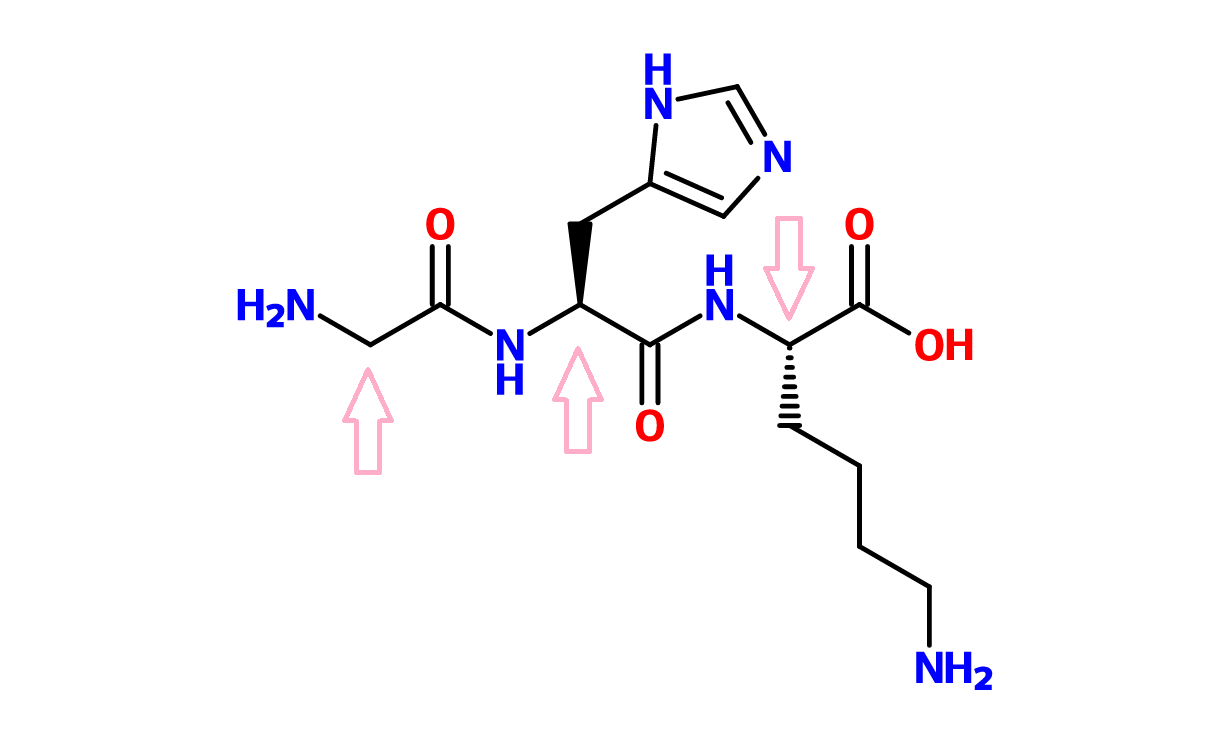
7. AMINO ACIDS
Amino acids are building blocks that link together to form proteins. Hydrolyzed amino acids are often used as conditioning ingredients for hair or skin. For example, GHK-Cu has been shown to have skin firming properties (source)
Figure 13. General structure of an amino acid.
Figure 14. GHK (Glycine-Histamine-Lysine)-Cu. The arrows indicate the central C of each amino acid.
8. BENZENE (ARYL)
A special, cyclic structure containing six C's and six H's with alternating double bonds (pi bonds). If the benzene ring is acting as a functional group off of a larger chain, then it's referred to as an aryl group. Methylparaben is one of the most common preservatives in cosmetics.
Figure 15. Benzene
Figure 16. Methylparaben
Stay tuned for some more lovely chemical structures, tomorrow!